At a recent symposium at Columbia University in New York, global leaders in biology, engineering, and material technology gathered to spawn what promises to be a transformative project with relevance to basic science research, regenerative medicine, oncology, drug development, and numerous other applications. This project has been named the MechanoMedicine (1) initiative and aims to integrate the burgeoning knowledge of cellular and molecular mechanics with advances in nanoscale manufacturing to create a new modality for studying and manipulating biological systems. In other words, researchers aim to leverage the mechanical properties of molecules and cells into a new avenue from which we can approach studying and treating disease.
Over the past decade the nascent but promising domain of research known as mechanobiology has blossomed into a full-fledged subject with an active community of engaged scientists and a growing clinical relevance. While there have been some truly remarkable discoveries in the field, most of the information gleaned has been too preliminary to integrate into medical therapies or technologies.
However, many now believe that this realm of knowledge is at a tipping point, and that it’s time to start looking for ways to apply the lessons learned in laboratory settings to medical applications. Along these lines, many of the speakers at Columbia not only reviewed the recent advances they had achieved, but also peered into the future to see where the field would be in the coming years.
In this article I'll give you a brief primer on the past decade of mechanobiology, and share some of my favorite take-aways from the MechanoMedicine symposium that will give us an inkling of what will be possible as the field advances.
That biological systems respond to physical stimuli is an obvious but mostly overlooked aspect of physiology. Our sense of touch is one clear manifestation of our physical stimuli response. Touch sensation is mediated by the opening of tension-gated ion channels embedded in touch neurons, which send signals to the brain in response to deformations of the cell membrane. Our other mechanical sense, hearing, is mediated in much the same way, with pressure waves bending tiny cochlear hairs to open channels in nerve endings. As these were the most obvious instances of physiological mechanical responses, they were among the first studied. The primary mystery at the time was how cells turn mechanical signals into chemical or electrical signals in a process that has been called mechanotransduction. As many groups would show in the following years, mechanobiology is by no means confined to mechanically activated channels, or even to the mechanisms of our conscious senses.
Breakthroughs in Mechanobiology
One of the most exciting discoveries of mechanobiology came out of the Discher lab at the University of Pennsylvania. In the journal Cell in 2006 (2), researchers showed that the terminal differentiation of stem cells could be guided by the rigidity of the substrate on which they’re grown. For example, growing stem cells on stiff surfaces directs them toward a bone cell lineage. As both stem cell biology and mechanobiology were fairly young fields at the time, this was a tremendous breakthrough. The paradigm in stem cell biology to that point was that embryos directed the maturation of stem cells by sending out chemical signals in a very precise way that varied in direction and time. By setting up gradients of the right molecules, the developing organism could establish its dorsal/ventral and lateral axes so that stem cells in different parts of the embryo would know what type of cell to turn into. The Discher group was able to direct stem cell differentiations in the absence of any chemical signaling. They showed that on rigid surfaces, stem cells would begin to take on the characteristics of bone cells; on soft surfaces, they would act like neurons; and intermediate stiffness would direct the cells toward muscle cell characteristics. The involvement of mechanical signaling solved an evolutionary quandary in developmental biology. If extremely precise control of chemical signaling in time and space is required, then any small perturbation during the developmental period would result in defects. With the added factor of mechanosensation, control over stem cell differentiation need not be so precise. Once the tissue types are established, a stem cell can know where it is in the body and what it should do simply by testing the elasticity of its surroundings, adding to the robustness of the developmental process.
Substrate elasticity directs stem cell differentiation. Blue, green, and orange symbols represent the level of proteins associated with brain, muscle, and bone cells, respectively. As substrate stiffness increases stem cells differentiate toward the cell type that resides in tissues of that elasticity. Blebbistatin is a drug that blocks the action of myosin motor proteins. When blebbistatin is applied to stem cells, they can't "feel" the substrate elasticity through the actin cytoskeleton, and therefore don't differentiate on any stiffness. Taken from reference 2.
Another breakthrough in mechanobiology came in 2009, again published in the journal Cell, this time from the lab of Valerie Weaver, now at UCSF (3). Since mechanical factors had been shown to be crucial in development and cell fate decisions, and as cancer can be seen as an aberrant developmental process, determining the role of mechanical signaling in cancer was intriguing. Papers dating back to 2004 link mechanical factors to cancer progression, but Weaver’s study offered the first direct, elegant, and definitive example of rigidity sensing in cancer development.
The team used breast cells with an inducible gene for a growth factor receptor (GFR). These receptors sit on the cell surface and look for signals to grow and divide, which come in the form of various growth factors secreted by other cells. Increasing the number of GFRs in a cell can turn a very small growth signal into a huge growth response, and over-production of GFRs is found in a significant portion of breast cancers. In fact, GFRs were one of the first causal mechanisms studied and understood in detail in the context of breast cancer (4). For their experiments, the researchers took the inducible GFR breast cells and grew them in culture until they formed acinar structures (small sac-like cavities) such as would ordinarily be found in mammary ducts. The researchers then turned on the GFR gene and substantially boosted the growth signal received by these cells. They found a disorganization of the acini, but surprisingly, no uncontrolled outgrowths.
To understand what happened next, we need to take a brief detour to learn a bit about tissue organization. Most cells in your body exist embedded in what’s known as an extracellular matrix (ECM). This is mostly made up of collagen and other long, fibrous proteins that are linked together to provide mechanical support and anchoring for the cells in the various tissues in your body. In some pathological conditions the composition and properties of the ECM are altered. This is actually what you’re checking for when you feel for lumps in the breast tissue; the dysregulation of ECM proteins causes a stiffening of the tissue.
After seeing no spreading cancer from the cells over producing GFRs, the researchers looked at what happened when they increased the rigidity of the ECM the cells were grown on. Again, they saw defects in the acinar organization, but no further cancerous phenotype. However, when they simultaneously increased the ECM stiffness and overexpressed GFR, they saw the acini break down entirely and the cells start to spread, just as migrating tumor cells break away from a primary structure to form metastases. Evidently, both the mechanical and chemical signals were required to transform the cells into an aggressive phenotype.
Matrix stiffness leads to cancer progression. ErbB2 is a growth factor receptor that is amplified in many breast cancers. In this experiment ribose treatment leads to ECM stiffening. Either GFR amplification or ECM stiffening breaks down the organization of acinii, but both are required for metastatic outgrowth. Taken from reference 3.
Mechanisms of Mechanotransduction
Clearly, cells are sensing and responding to their mechanical environment, but how? One might take the lessons from touch and hearing and guess that ion channels are the means of conveyance of most mechanical signals. Many scientists guessed just that, but it turns out not to be the case. Those channels cause transient currents when they open, so they’re good at detecting dynamic forces that occur on short time scales, but they’re less well adapted for sensing a static input, such as the rigidity of a tissue.
It turns out that many proteins change their shape when subjected to force. Cell shape and structure are defined by a dynamic cytoskeleton. One of the components of this cytoskeleton is the filamentous protein actin, which you may recognize as the primary structural component of muscle cells. In non-muscle cells, actin is connected to the ECM through a series of adaptor proteins that transmit force as the actin is being tugged and remodeled by motor proteins within the cell.
The Sheetz and Fernandez groups at Columbia University have been instrumental in showing that many of these adaptor proteins have a force threshold over which they rearrange their structure. This can form new protein-protein binding sites, reveal formerly buried amino acids for chemical modification, or expose peptide sequences for cleavage by proteinases. As these mechanosensitive proteins are being pulled by actin, the elasticity of the ECM will determine whether they reach these force thresholds that initiate downstream signaling events.
Molecular Scale MechanoMedicine
More by James
One session focused on MechanoMedicine at the molecular scale. Highlights included the nuclear aspects of mechanotransduction, with research on mechanically directed protein shuttling to and from the nucleus (5), and the epigenetic changes that are driven by mechanical signals (6). Another topic was technologies being developed to probe the mechanical properties of biomolecules. Much of the work done in this arena is accomplished by modifying atomic force microscope tips for the attachment of proteins, a process termed biofunctionalization (7). This allows for very precise (picoNewton) measurements of force and the corresponding changes in protein structure. Another exciting technology that looks like it's just about ready to come down the pipeline is nanoscale patterning of surfaces with bio-active molecules(8). This will allow researchers to investigate the effects of specific geometries and sizes on mechanotransduction. By placing a bounds on the minimum feature that cells are able to detect we'll gain a better idea of the complexity of the molecular structures involved in mechanosensation.
Acting as a simple pump, the heart is the most mechanical organ. With an aging population across the developed world and heart disease the leading cause of mortality in these populations, pathologies of the heart and vasculature are perhaps the greatest opportunity for clinical translation of mechanobiology. Adam Engler (the primary scientist who showed that stem cell differentiation can be determined by substrate rigidity) and his group were able to identify vinculin, one of the anchoring proteins that link actin to the ECM, as a key to healthy heart contractions in aged hearts (9). Dr. Engler described how his group had developed and characterized flies for investigations into the mechanical properties of the aging heart (the proteins that make up the fly heart are 82% similar to those in human hearts). These studies and others like them being carried out in a number of other labs have the potential to reveal more druggable targets in the fight against heart disease.
Cellular Scale MechanoMedicine
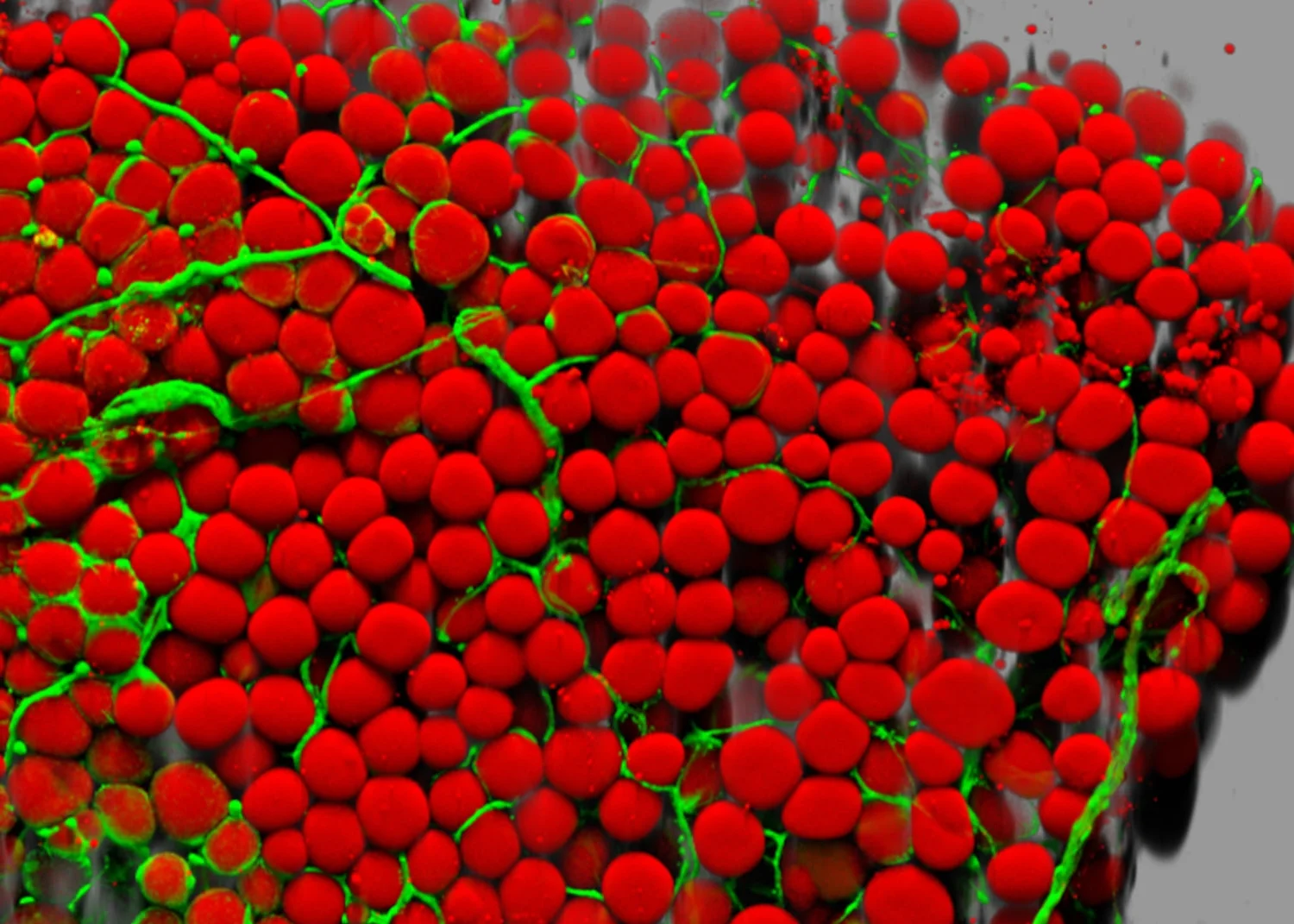
Other exciting projects focus on the whole cell rather than its parts. Topics included the mechanical requirements for immune cell function (10), the mutations in mechanosensative machinery in brain cancer (11), mechanotransduction of force loading in bone (12), and the critical roles of mechanical signals in engineering functional human tissues (13). One unique insight presented at the cellular session was that cells don’t only respond to mechanical stimuli, but also have mechanical properties of their own that we can utilize for diagnostics and manipulate for therapies. A particularly promising project in this vein takes advantage of the fact that cancer cells have distinct sizes and densities from the rest of the cells in the bloodstream. Researchers from the University of Singapore, led by Chwee Teck Lim have devised a microfluidic device to isolate these so-called circulating tumor cells (CTCs) from blood samples (14). This will prove hugely useful in both basic science research and diagnostics. For basic science, characterizing the population of CTCs and then relating it to patient outcomes can teach us more about the causes of cancer metastasis. Is it just a numbers game, with a greater load of CTCs directly correlating to faster metastasis? Are there specific subtypes of CTCs that are more likely to embed in certain tissues? Is the population of CTCs clonal (genetically identical) or is there a heterogeneous population? Are there any specific classes of mutations in CTCs that lead them to be more or less aggressive? This technology can answer all of these questions. Once this body of knowledge is established, doctors will be able to look at a patient’s CTCs and direct their treatment options. Some patients will need a more aggressive course of chemotherapy to stand a chance at remission, and some will be able to avoid the harsh side effects of certain therapies if their particular CTC population is resistant against that anyway. As the general field of cancer biology moves forward, this will provide huge synergies with such a technology.
Circulating tumor cells have different sizes and densities than white blood cells. This microfluidics device uses these properties to separate out CTCs from a blood sample for genetic characterization. Taken from reference 14.
Tissue Scale MechanoMedicine
Many see the next step in mechanomedicine as linking what is known about mechanobiology into the much larger body of knowledge in fields such as endocrinology and immunology, and this was on full display at MechanoMedicine 2015. Linking with an earlier talk on mechanotransduction in bone cells, researchers presented on the role of estrogen signaling in skeletal mechanobiology (15), showing the crosstalk between hormone signalling and mechanical signalling. Other groups focused on the intersection of neurology and mechanobiology. The first used ultrasound to probe and modify the blood-brain barrier (BBB). Ultrasound is not just useful as an information-gathering tool, but can be used to manipulate biological tissues. One of the great difficulties in treating neurological conditions is that very few molecules and almost no drugs can cross the BBB, necessitating highly invasive methods for treating many conditions. Elisa Konofagou and her lab at Columbia have shown that they can open the BBB transiently, locally, and non-invasively using focused ultrasound, which promises to be a boon for drug delivery to the brain (16). Barclay Morrison, also of Columbia, uses high-speed imaging, among other tools, to study traumatic brain injury. He presented data specifically relating to blast trauma from improvised explosive devices. Hopefully the relevance of this specific trauma will decrease moving forward, but the lessons learned will be applicable to injuries from athletics and road accidents. Any tech geeks should visit his lab website (17), as he manages to hit all the buzzwords: high-speed video, carbon nano-tubes, micro-electrode arrays, etc. In a talk that showed the greatest integration both horizontally across fields, and vertically from molecular to tissue level, Arthur Mak of Chinese University of Hong Kong discussed the mechanical damage that can occur in heart tissue when it is under oxidative stress (18). Mak once again touched upon the relevance of mechanobiology to cancer, with an investigation into the role of fluid flow forces in angiogenic sprouting: the formation of new blood vessels that supply tumors with nutrients (19).
The talk that might have the widest clinical applicability, however, was an investigation into the biomechanics of the cervix (20). Pre-term birth is the leading cause of mortality worldwide in children under 5, and is caused by defective tissue integrity in the mother’s cervix. The cervix is a dense meshwork of collagen and smooth muscle cells, and it remodels constantly throughout pregnancy and birth depending on both chemical and mechanical cues. A better understanding of what fails in these signaling pathways leading to a weak cervix and pre-term birth would help identify susceptible individuals early in the process and target intervention, and potentially in the future develop treatments, whether drugs or purely mechanical devices, to prevent the cervical failure.
The physical properties of the cervix change throughout a healthy pregnancy. Differences in the rate or extent of these changes can lead to preterm birth. Understanding the mechanisms of these differences will allow doctors to screen for at-risk pregnancies before they become a problem. Taken from reference 20.
Future Technologies and Moonshots
For the vast majority of modern medical history, our arsenal of drugs has consisted of a library of small molecules, which often act through unknown mechanisms. Recent advances have allowed us to add biological agents which are much more directed in their actions, most notably monoclonal antibodies and viral vectors for gene therapy, to our medical toolbox. Adding a third avenue through which we can direct therapies will give us exponentially more combinations of strategies when confronting disease. Combined with advancing technology that makes genetic sequencing available to everyone’s doctors for personalized medicine, this promises to usher in an era of previously unimaginable precision in targeted therapies. As with any endevour aiming to push the boundaries of knowledge, the vast majority of these projects will either fail or never reach the level of efficacy required for broad application, but with so many talented researchers making a push to transition from mechanobiology to mechanomedicine, it’s only a matter of time until these technologies start making a difference in a clinical setting.
References
- http://engineering.columbia.edu/nsf-symposium-kicks-launch-new-columbia-mechanomedicine-center
- Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. "Matrix elasticity directs stem cell lineage specification". Cell 126, 677–689 (2006).
- Levental, K.R., Yu, H., & Kass, L., et al. "Matrix crosslinking forces tumor progression by enhancing integrin signalling". Cell 139, 891–906 (2009).
- Hudziak, R.M., Schlessinger, J., & Ullrich, A. "Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenisis of NIH 3T3 cells". PNAS 84, 7159-7163 (1987).
- Gregg Gundersen (Columbia University)
- Yingxiao Peter Wang (UCSD)
- Julio Fernandez (Columbia)
- Shalom Wind (Columbia)
- Kaushik, G., Spenlehauer, A., et al. "Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart". Science Trans Med 92, 292ra99 (2015).
- Lance Kam (Columbia)
- Sanjay Kumar (UC Berkeley)
- Christopher Jacobs (Columbia)
- Gordana Vunjak-Novakovic (Columbia)
- Warkiani, M.E., Guan, G., et al. "Slanted spiral microfluidics for ultrafast, label-free circulating tumor cells isolation". Lab on a Chip 14, 128-137 (2014).
- Marjolein van der Meulen (Cornell University)
- Elisa Konofagou (Columbia)
- Barclay Morrison (Columbia)
- Arthur Mak (Chinese University of Hong Kong)
- Roland Kaunas (Texas A&M)
- Badir, S., Mazza, E., Zimmermann, R., and Bajka, M. "Cervical Softening Occurs Early in Pregnancy: Characterization of cervical stiffness in 100 healthy women using the aspiration technique" Prenatal Diag. 8, 737-741. (2013)