Despite the brain’s centrality to everything that makes being human interesting, we know fairly little about how the brain works at a detailed circuit level. This is because the human brain is astonishingly complex. An adult brain has about 100 billion neurons, each making hundreds or thousands of connections to other neurons. The complete connectome (total number of connections, or synapses) is estimated to number in the hundreds of trillions (1,2). However, we have the good fortune of living in a time where the basics of neuronal function are now well understood, and tools are being developed to investigate how they link up into the complex circuits that make our thoughts, emotions, and actions. In April 2013, the White House announced the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative. Despite the clumsy acronym, this is a truly transformational project that aims to devote a sum of $300 million to research that seeks to understand the nervous system and associated pathologies. Psychiatric diseases, Alzheimer’s, Parkinson’s, and autism are each estimated to cost the US economy in the tens or hundreds of billion dollars every year (3). If this initiative can put even a 1% dent in any of those numbers, it would be a tremendous return on investment.
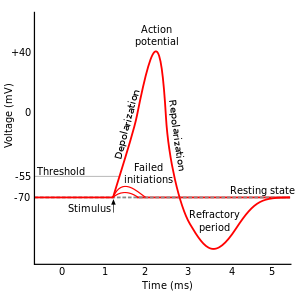
The most fascinating and promising of these tools has the moniker optogenetics. As you might surmise from the name, this technique uses light and genetic manipulation to control neurons. In order to understand how, we first need to know a little about how neurons work. In a resting state, neurons use pumps to move charged particles, or ions, to maintain a voltage across the outer membrane of the cell. They generally keep the membrane polarized at around –70 millivolts, and this is referred to as the resting potential. In addition to ion pumps, which use energy to precisely control the movement of charged particles, neuronal membranes also have ion channels, which allow passive diffusion of ions when they are open. While these channels lack the ability to move ions against a concentration gradient, they allow for much greater speed of ion flow. The class of channels that allow neurons to work are voltage-gated ion channels. These open and close based on the membrane voltage, and are kept closed at the resting potential. Inputs to a neuron will perturb the resting potential, either inhibiting the neuron by pushing the membrane potential further negative (hyperpolarizing inputs), or exciting it by bringing it closer to 0 (depolarizing inputs). Each class of neuron has its own type of voltage-gated channels, but they all open somewhere between the resting potential and 0 potential. Therefore, if a neuron receives enough depolarizing inputs, they will push it toward 0 membrane potential and trip the threshold for opening voltage-gated channels. When these open, positively charged sodium ions rush into the cell, pushing the membrane potential positive in an event called an action potential. This action potential will then open the voltage-gated channels in the adjacent areas of membrane and hence propagate down the length of the neuron as a wave of positive ion flux leading to downstream events such as the release of neurotransmitter molecules.
The basics of action potential firing in single neurons have been well understood for some time. However, how these neurons link up into circuits and networks that all feed back on one another with inhibitory hyperpolarizing inputs and excitatory depolarizing inputs is a intricate and difficult system to study due to its scale and complexity. Generally, when we’re trying to learn about a complex system we don’t understand, a good first method is to fiddle around with the components and see what happens. That’s where genetic techniques come into play. By playing with the genetics of model organisms like worms, flies, or mice, we can get different classes of neurons to synthesize different types of ion pumps and channels. Since proteins involved in ion trafficking are so fundamental to cell function, they are found ubiquitously in all forms of life. This gives researchers a tremendous array of off-the-shelf components to try in our model organisms. By further engineering these proteins, we can have even more precise control over how they function. These sorts of methods have been employed to study the role of different classes of neurons in a range of behaviors in the model systems with very interesting results, but they present a problem in that they’re rather blunt instruments. The inserted pumps and channels can be manipulated by drugs, or can be designed to be temperature-sensitive, but when you’re altering the characteristics of entire classes of neurons at once, it’s hard to determine the fine-grained details of what’s happening on a cellular level in the brain. As an analogy, imagine you’re studying the dancing behavior of a group of shy people (picture your stereotype of a neuroscientist in a white lab coat). If your two experimental conditions were to have everyone entirely sober, or everyone so drunk they can’t stand, you wouldn’t see any dancing and you’d think that alcohol doesn’t have very much effect on dancing, but you’d be missing all of the interesting behavior and interactions that result between the two extremes when you have a bunch of different people at varying levels of sobriety. Additionally, neural circuits are dynamic systems that operate on millisecond time scales. Temperature changes and drugs are not only blunt instruments spatially, but can’t be modulated faster than a scale of minutes or hours. If we had to wait for our neuroscientists to sober up for every new experiment, we wouldn’t be able to get very much done.
So what we need, in order to study the brain at finer detail and faster time scales, is a way to change the excitability of neurons in smaller scales (distribute the tequila to specific neuroscientists in our dancing example), rapidly, and reversibly. This is where the optics comes in. A family of proteins called opsins has the interesting property that they change their structure when stimulated with light (these are the operative proteins in your retina that allow for vision). It was found that a number of microbial species have opsins incorporated into ion channels to control their opening. Therefore, just as in normal neurons action potential firing is controlled by voltaged gated ion channels, we can genetically engineer neurons such that action potential firing is also controlled by light gated ion channels. Focused laser light can be used to activate or suppress these neurons in very small, precise areas of the brain, giving researchers unprecedented resolution in studying neuronal circuits.
A neuron with a ion channel gene inserted to cause a depolarizing current on stimulation with yellow light fires an action potential when illuminated. Original image taken from UCI Research. Modified by the author.
Even though this is a very young technology, its power was immediately realized and hence it has been applied to many pressing research questions. Optogenetics has been utilized to study the circuits involved in fear conditioning and anxiety (4), Parkinson’s disease (5), circadian rhythms (6), and addiction (7), among many others. Most of the studies thus far have looked at one type of light-gated ion channel in a particular subset of neurons. What makes this technology even more exciting is the potential for simultaneous multiplexed optogenetic manipulations in single systems. These opsins have already been tuned to respond to a variety of light wavelengths, extending into the infrared (IR). IR activation will prove extremely helpful for certain brain structures, as long wavelength photons penetrate deeper into tissue than visible light. Early genetic studies of neuronal circuits gave us clues as to where to look in the brain for interesting phenomena related to neuropathies. By combining a variety of different channels (excitatory and inhibitory) in varying neuronal classes, all driven by different wavelength light in the same brain researchers will have exquisite control over many aspects of the neuronal circuitry that they can use to probe those aspects that are still mysterious. The future of optogenetics appears to be very bright indeed.
Further Reading:
For a first hand account of the development of optogenetics by one of the pioneers of the the technique check out http://f1000.com/prime/reports/b/3/11
For a cool video animation of firing neurons check out http://video.mit.edu/watch/optogenetics-controlling-the-brain-with-light-7659/
1) Interestingly these numbers are actually larger in toddlers. Most of what we think of as brain development in childhood and adolescence is not the growth of new neurons and formation of new synapses but the pruning of existing structures and fine tuning of those that persist.
2) http://www.neurology.org/content/64/12/2004
3) http://mcgovern.mit.edu/brain-disorders/by-the-numbers
4) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2906568/
5) http://www.sciencemag.org/content/324/5925/354
6) http://www.ncbi.nlm.nih.gov/pubmed/25643294?dopt=Abstract
7) http://www.cell.com/cell/abstract/S0092-8674%2815%2900004-