Last month, newspapers rushed to report that, for the first time, a woman in the United States carried bacteria resistant to last-resort antibiotics. Researchers originally identified the so-called “nightmare” bacteria in pigs, raw pork, and a small number of individuals in China. They watched as the superbug traveled to Europe and then onward to the United States. The presence of this superbug on our shore breeds some serious questions: how do superbugs arise? How did they spread so quickly? What are their implications on human health? Most importantly, what can be done to stop their growth and spread?
A “superbug” refers to any bacteria resistant to at least one class of antibiotic [1] . This past year, the Center for Disease Control (CDC) reported that superbugs infect approximately two million people in the United States each year – and 23,000 people have died from a result of these infections. At this point, some classes of antibiotics have become so widely useless due to resistance that they are no longer prescribed to treat their original bacterial target [2].
The main reason bacteria can acquire resistance so quickly is because of their remarkable replication rate. It takes a mere 17 minutes for a colony of E. coli cells to double in size. Every time a bacterium splits, it must replicate its entire genome to pass to its successor. Bacteria are not particularly good at making perfect replicas of their genome, and tend to leave a few changes scattered throughout. Sometimes, these changes--called mutations--can help a bacterium to resist antibiotics.
Bacteria that happen to carry drug resistance mutations will survive when they’re faced with that drug, and after treatment, only those resistant bacteria will survive and then multiply. Drug resistance can therefore spread virtually any time bacteria are exposed to drugs that would destroy or limit their growth; hence, it is important that antibiotics are used with prudence. We can easily imagine restricting antibiotic use by encouraging caution in prescribing antibiotics in doctors’ offices. But there is an enormous and surprising source of antibiotic use - factory farming - that may explain why the first rare strains of the nightmare superbug appeared in pigs.
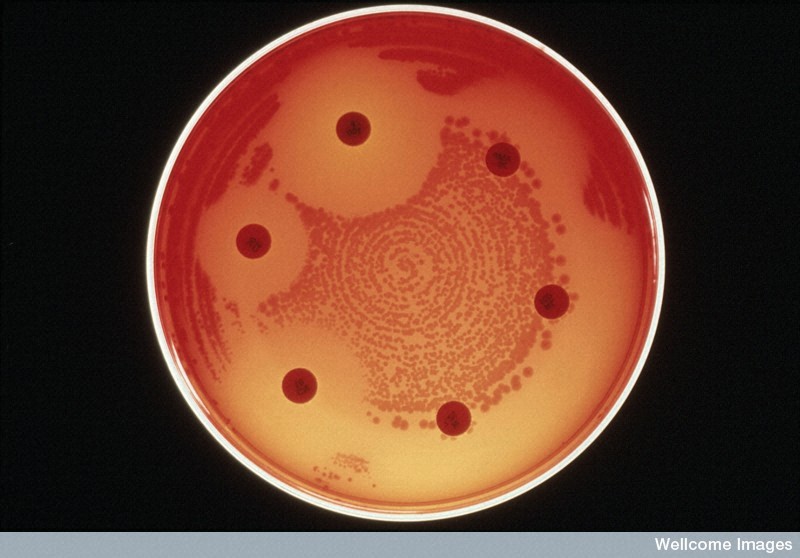
Differing levels of drug resistance in bacteria. Antibiotics (large pills) can inhibit the growth of bacteria (small dots) to varying degrees. Bacteria that can grow right next to the antibiotic pills are resistant to that antibiotic.
A 2009 study by the FDA revealed that 27.8 million pounds of antibiotics are used on livestock every year in the United States [4]. Antibiotics on factory farms are used daily for two primary purposes: first, to ward off the pandemic potential caused by holding animals in close quarters, and second, to increase weight gain in animals. In the 1950s, scientists discovered that continuous low-dose antibiotics caused animals to gain more weight per pound of feed [5], which we now know is due to changes in their gut microbiome in response to the antibiotics [6]. Farmers exploit this relationship between antibiotics and animal weight to fatten livestock and decrease the time needed for an animal to reach its target weight. In 2009, the FDA estimated that 39% of animal antibiotic sales were not used for immediate medical concerns [4].
“Antibiotics on factory farms are used daily for two primary purposes: first, to ward off the pandemic potential caused by holding animals in close quarters, and second, to increase weight gain in animals.”
There is strong evidence that the use of antibiotics in animals leads to increased antibiotic resistance in bacteria that live in the animals--and that can also infect humans. In 2012, researchers showed that methicillin-resistant Staphylococcus aureus bacteria (MRSA) emerged from livestock treated with antibiotics [7]. Drug resistance in E. coli is also tied to antibiotics used in poultry [8]. Another 2012 study found large numbers of antibiotic resistance genes in swine farms in China [9].
“The use of antibiotics in animals leads to increased antibiotic resistance in bacteria that live in the animals—and that can also infect humans.”
These resistant bacteria hitch rides into human civilization. Bacteria can hide in meat and animal products sold in grocery stores, jump onto the farmers who work with animals [8], and even enter the water supply via runoff from soil [10]. A farmer who over-treats his or her animals with antibiotics is thus at high risk for becoming a superbug host. From these initial sources, the bacteria spread among humans and disseminate their drug resistance. The bacteria can also hitch rides on other animals; in one 2008 study, scientists found drug-resistant E. coli in birds as far away as the secluded Arctic tundra [11].
It should be noted that 30% of the antibiotics used on farms, called ionophores, are not used in human medicine, and might not seem like a risk to people [4]. Yet ionophores can still lead to bacterial resistance against human medications. This resistance appears because bacteria can mutate in ways that make them resilient against broad classes of medications, including those used in humans. For instance, they can develop molecular “pumps” that push out antimicrobial agents, which might be useful against both ionophores and some human medications.
“These resistant bacteria hitch rides into human civilization. Bacteria can hide in meat and animal products sold in grocery stores, jump onto the farmers who work with animals, and even enter the water supply via runoff from soil.”
Once they acquire these broad resistance mechanisms, many bacteria also have the ability to pass this resistance to other bacteria by directly trading genes. An early paper showed that treatment of chickens with tetracycline, a human antibiotic, resulted in increased resistance against multiple classes of antibiotic drugs [12]. Another study showed that treatment of cattle with one common ionophore led to a 32-fold increase in resistance against an antibiotic used in humans [13].
“Once they acquire these broad resistance mechanisms, many bacteria also have the ability to pass this resistance to other bacteria by directly trading genes.”
Regulation of antibiotic use in the farming industry is limited. The FDA and Health Canada both recently changed industry guidelines to phase out antibiotic use for growth purposes; however, compliance with such guidelines is only considered voluntary. As of October 2015, California became the first state to set strict standards for antibiotic use in animals, although the bill will not go into effect until 2018.
In contrast, the EU officially banned the use of antibiotics for growth purposes in animals in 2006. Research showed that reductions in antibiotic use for growth promotion in the EU led to a significant drop in antibiotic resistance, with minimal effects on animal health [14].
In 2015, the authors of a study published in the Proceedings of the National Academy of Sciences projected that there will be a 67% increase in global antimicrobial consumption by the year 2030 [15], driven by the growth of industrializing countries. Without global efforts to regulate antibiotic use, this growth could lead to vast increases in multi-drug-resistant bacteria. In the United States, we have just encountered our first bacteria with resistance to a last-resort drug. With this resistance we can expect more hospitalizations, more money and medical resources spent, and more deaths from diseases once considered curable.
Sources
1. “Antibiotic/Antimicrobial Resistance.” Centers for Disease Control and Prevention. 2 Mar. 2016. Web. 12 Mar. 2016.
2. Wellems TE and Plowe CV. “Chloroquine-Resistant Malaria.” The Journal of Infectious Diseases: 2001; 184(6); 770-776.
3. Sender R, Fuchs S, and Milo R. “Revised estimates for the number of human and bacteria cells in the body.” Preprint on bioRxiv. http://dx.doi.org/10.1101/036103 (2015).
4. Food and Drug Administration. “2009 Summary Report on antimicrobials sold or distributed for use in food-producing animals.” Dec. 2010.
5. Groschke AC and Evans RJ. “Effect of antibiotics, synthetic vitamins, vitamin B12 and an APF supplement on chick growth.” Poultry Science. 1950; 29(4); 616-618.
6. Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. “Antibiotics in early life alter the murine colonic microbiome and adiposity.” Nature. 2012; 488(7413); 621-626.
7. Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, et al. “Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock.” mBio. 2012; 3(1); e00305-11.
8. van den Bogaard AE, Driessen C, and Stobberingh EE. “Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers, and poultry slaughterers.” Journal of Antimicrobial Chemotherapy. 2001; 47(6); 763-771.
9. Zhu Y, Johnson TA, Su J, Qiao M, Guo G, Stedtfeld RD et al. “Diverse and abundant antibiotic resistance genes in Chinese swine farms.” Proceedings of the National Academy of Sciences. 2012; 110(9); 3435-3440.
10. Martinez-Carballo E, Gonzalez-Barreiro C, Scharf S, and Gans O. “Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria.” Environmental Pollution. 2007; 148(2); 570-579.
11. Sjölund M, Bonnedahl J, Hernandez J, Bengtsson S, Cederbrant G, Pinhassi J et al. “Dissemination of multidrug-resistant bacteria into the arctic.” Emerging Infectious Diseases. 2008; 14(1): 70-72.
12. Levy SB, FitzGerald GB, and Macone AB. “Changes in intestinal flora of farm personnel after introduction of a tetracycline-supplemented feed on a farm.” The New England Journal of Medicine. 1976; 295(11); 583-588.
13. Houlihan AJ and Russell JB. “The susceptibility of ionophore-resistant Clostridium aminophilum F to other antibiotics.” Journal of Antimicrobial Chemotherapy. 2003; 52; 623-628.
14. Wegener HC. “Antibiotics in animal feed and their role in resistance development.” Current Opinion in Microbiology. 2003; 6(5); 439-445.
15. Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP et al. “Global trends in antimicrobial use in food animals.” Proceedings of the National Academy of Sciences. 2015; 112(18); 5649-5654.