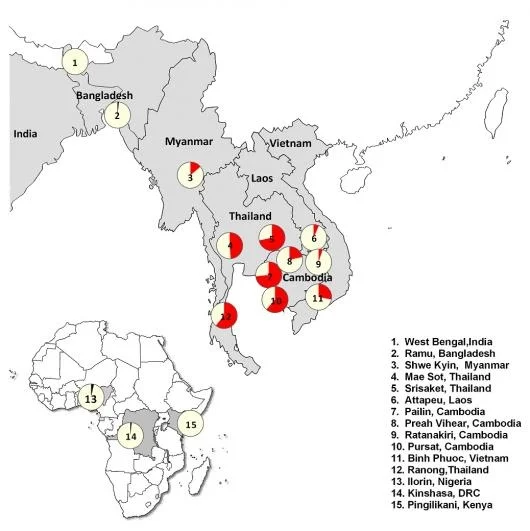
Just last week a report in the New England Journal of Medicine detailed the extent to which resistance to the current first-line antimalarial drug artemisinin has spread from its epicenter, the Thai-Cambodia border. This study conducted by researchers from different institutions, collectively known as the Tracking Resistance to Artemisinin Collaboration (TRAC), monitored artemisinin resistance from 2011 to 2013 across 10 countries — primarily in Southeast Asia, but also in South Asia and sub-Saharan Africa.
Currently, resistance to artemisinin is predominantly concentrated in Southeast Asia, but it is on the cusp of spilling over to more densely populated areas such as Bangladesh, India, and sub-Saharan Africa (where a majority of malaria deaths occur). Furthermore, there is no replacement antimalarial drug at this moment that would be ready to be deployed if resistance were to spread quickly. The situation is dire enough that senior author of the study Nicholas White penned an article in The Guardian proposing a seemingly impossible “all-out assault” in order to eliminate malaria before it spreads further.
Understanding Artemisinin Resistance
In the late 1990s to early 2000s, Southeast Asia adopted artemisinin combination therapies (ACTs), where artemisinin is paired with a longer-lasting partner drug. As ACTs were deployed, researchers concurrently monitored the efficacy of these drugs in the Greater Mekong subregion. Patients suffering from malaria would arrive at a clinic where their blood would be drawn and examined by microscopy for the presence of malaria parasites residing in their red blood cells. If positive, they would be given a drug treatment over 3 days, and their parasite burden would be monitored and recorded periodically until parasites were cleared, usually around the second day. Over time, researchers began noticing that more and more patients still had detectable parasitemias three days after their first drug treatment. This was the start of artemisinin resistance.
Artemisinin resistance, as defined by malaria researchers, is not a full clinical treatment failure. In other words, resistant parasites are eventually cleared from patients’ blood, but at a much slower rate than normal. This is often also referred to as “delayed parasite clearance.” To some, this may not sound terribly dangerous because people do eventually get cured of their infection.
However, two immediate points can underline the severity of the situation. The first is that we may only be at an intermediate step between drug sensitivity and full-fledged drug resistance that will manifest itself as clinical treatment failure. As we continue to apply selective pressure, these parasites will continue to evolve and find a way to survive.
According to WHO, 90% of the 2012 global malaria deaths were in Africa, predominantly children under the age of 5. This is despite the fact that ACTs have been deployed as the first-line antimalarial therapy in a majority of malaria endemic African nations since 2005-2006. Therefore, if resistance in its current form were to spread out of Southeast Asia to Africa, the 49% decrease in mortality caused by malaria in Africa since 2000 would be under high risk of reverting.
Setting a Precedent
Artemisinin may be on its way out as the first-line antimalarial as resistance spreads, but this type of story isn’t new to malaria elimination. In 1945, chloroquine was introduced as a highly-effective antimalarial and quickly became used around the world. One method of distribution was to incorporate it into table salt as a prophylactic measure in malaria-endemic regions so people would receive regular doses. These efforts, however, were out-maneuvered by parasite evolution, and in 1957 the first incidence of chloroquine resistance was recorded at the Thai-Cambodia border.
Within the next decade, chloroquine resistance spread throughout Southeast Asia. Over the following decade, it spread further west. By 1978, reports of chloroquine resistance surfaced in East Africa (Kenya and Tanzania). By the 1990s, resistant parasites overtook wildtype populations across sub-Saharan Africa. According to data collected by the WHO, the loss of chloroquine as a global first-line antimalarial led to an increase in hospital admissions, malaria epidemics, disease transmission, and ultimately mortality.
Getting Off the Evolutionary Treadmill
After chloroquine resistance became the norm around the world, significant efforts were made by WHO, the Global Fund, and other organizations to transition the world to ACTs. Today, as artemisinin resistance is likely to spread globally, many drugs are still not prepared for large-scale deployment. In the same issue of the New England Journal of Medicine, one drug KAE609 is the farther along the development path than most. However, KAE609 resistance itself may be inevitable and has in fact already been well-documented in laboratory experiments.
Current malaria vaccine trials, though promising, are too early to know if efficacy is long-lasting. Long-lasting insecticide-treated bed nets, which have contributed hugely to the decline in malaria deaths over recent years, are helping, but cannot eliminate malaria alone.
In order to eradicate malaria in the US, the Office of Malaria Control in War Areas, which became part of the CDC, made an enormous effort in mosquito vector control. They sprayed DDT in over 4.6 million homes, drained swamps, sprayed insecticides over large swaths of land, and destroyed many mosquito breeding grounds. People also began putting screens in their windows. The massive effort paid off, and the US was declared free of malaria in 1949.
Given the humanitarian and economic problems that artemisinin resistance may bring, Nicholas White is right in calling for an all-out assault.